Technology Centre Build Photography and Digital Arts Fda
OVERVIEW
FDACONSTRUCTION.WORDPRESS.COM TRAFFIC
Date Range
Date Range
Date Range
LINKS TO WEBSITE
Shoot One of Part Three. After a Double Shift at the Chip Shop. The amber-lit walk home grew ever-longer as his feet began to swell in his two-for-one shoes.
WHAT DOES FDACONSTRUCTION.WORDPRESS.COM LOOK LIKE?
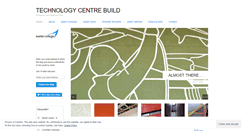
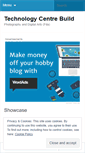
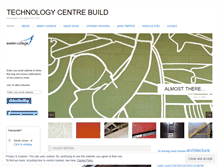
FDACONSTRUCTION.WORDPRESS.COM SERVER
BROWSER IMAGE

SERVER SOFTWARE
We discovered that fdaconstruction.wordpress.com is weilding the nginx os.HTML TITLE
Technology Centre Build Photography and Digital Arts FdaDESCRIPTION
Photography and Digital Arts FdaPARSED CONTENT
The site had the following in the homepage, "Technology is on the Way." I noticed that the web site stated " The weather continued to mistreat Peter this week, despite the elements best efforts, we have another set of 12 photographs for the blog." They also stated " Peter continues to take some great photographs and for your viewing pleasure here are this weeks."ANALYZE MORE BUSINESSES
FDA Regulatory and Quality System Consultants. Assisting Manufacturers and Distributors with FDA Regulatory. Services in USA, Asia and Europe. We deliver excellent value for your regulatory and quality. Assurance needs to market your medical devices and medical components. FDA Delays eCTD Requirements for Master Files and Biological Files. FDA set date for Electronic Submission. FDA draft guidance for Medical Devices.
Smith Associates, FDA Consultants. Consultants Specializing in Regulatory Affairs for FDA Compliance. Smith Associates is located 20 minutes from FDA enabling quick response to FDA during interactive reviews.
FDA and International Consulting and Training Services. So Cal - Tourist Info. Medical Device Training to be held at Disneyland Resort! Expert Consultants ready to assist with your compliance needs! Medical Device Regulatory Submissions cleared quickly! Over 25 years of hands-on experience allows us to help our clients navigate the regulatory and compliance issues faced on a daily basis as well as occasional unwanted surprises encountered.
In 2016 we formed a partnership with Jennings O Donovan and Partners Ltd.
Solving FDA Legal Challenges for the Life. Of a Life Sciences Company. How we help solve FDA legal challenges. For the life of a life sciences company. Occasional musings on new developments. In the regulation of life sciences companies. Law Offices of Michael A. 12519 El Camino Real, Suite E, San Diego, California 92130.